Covid-19 vaccines: What other countries have done and what Americans can expect
December 11, 2020
Since March of 2020, numerous pharmaceutical companies have researched and developed Covid-19 vaccines. Many have entered Phase 3 of testing their vaccines. With that in mind, countries are beginning to move forward.
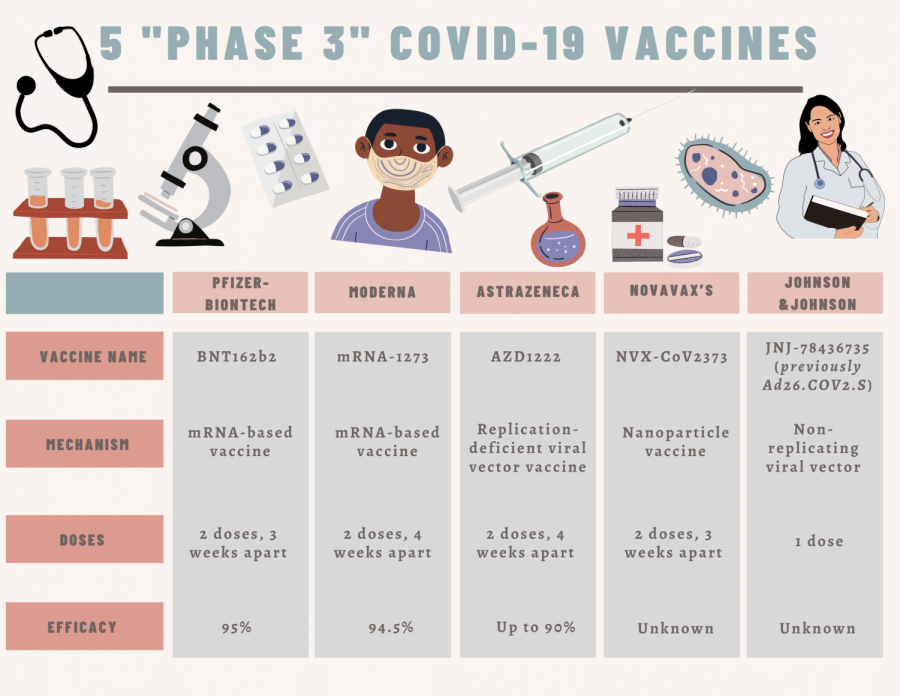
The Pfizer-BioNTech and Moderna vaccines are the first two vaccines in the process of getting FDA emergency authorization. Countries such as Canada and the U.K, however, have already approved and begun distribution of those vaccines. These two vaccines are different than many others, as they are the first ones to use mRNA, according to Renee Ogle, an AP Biology teacher at Amador.
“They inject mRNA for the spike protein on the surface of the virus so that your body will recognize the protein, so that your T cells can fight the virus without you getting sick. Then you would make B cells so that you would recognize the virus in a future exposure,” said Ogle.
However, in the U.S., nothing has been authorized yet, according to Kerri Leedy, a PR and Media Relations Manager for Kaiser Permanente in northern California.
“No COVID-19 vaccine has yet been approved for distribution in the U.S,” said Leedy.
As for who the priority recipients of the vaccine will include, rules will be set in order to determine this.
“There will be limited supply available when the first vaccine is approved. The CDC will oversee COVID-19 vaccine distribution in the U.S. and issue guidelines for prioritizing those populations that should receive the vaccine first. Federal officials have said that health officials in each state will have the ultimate authority to prioritize populations in their state,” said Leedy.
The U.K plans to offer the first approved batch of vaccine to priority groups, which include health and social care staff, as well as individuals that are at a greater risk of exposure to the virus. Additionally, the U.S intends to go by similar guidelines.
“Healthcare workers will be among those prioritized, along with people most vulnerable to severe illness from the virus, such as those living in skilled nursing facilities,” said Leedy.
Essential workers are regularly exposed to individuals due to their jobs. While there are differing opinions, some of them might consider taking the COVID-19 vaccine, including Robert, a cashier at Fiesta Taco.
“I would take it because that’s the only way to stop the spread so everybody will be safe. I want to be safe and it will be good for everyone and to stop the spread,” said Robert.
Yesterday the FDA panel voted in favor of recommending Pfizer-BioNTech’s vaccine for emergency distribution, bringing us a step closer to the CDC’s goal to making the first supply of vaccines available by the end of 2020.